Specific monomer sequences are essential for the development of cationic polymers that can adhere to negatively charged surfaces in saline environments. We show that copolymers with adjacent cation–aromatic sequences can be synthesized through cation–π complex-aided free-radical polymerization, which exhibit fast, strong, but reversible adhesion.
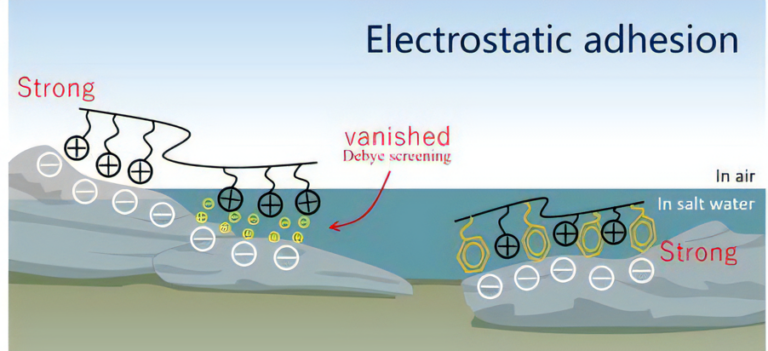
Many solid surfaces, including rocks, glasses, and metals, are negatively charged in marine environments. However, adhesion based on electrostatic interactions in such conditions normally diminishes due to the Debye screening effect (left in the image). Biosystems can use this interaction through adjacent cationic–aromatic amino acid sequences of proteins even in a saline medium (right in the image), which provides a molecular design model to develop marine adhesives [1]. However, sequence-controlled polymerization is still a central challenge in polymer chemistry [2].
In our study, we have discovered that a series of copolymers bearing adjacently located cationic and aromatic residuals, hereafter referred to as poly(cation-adj-π) (adj is short for adjacent and for aromatic monomer), can be synthesized in abundance through cation–π complex-aided free-radical polymerization from cationic and aromatic monomers at an equimolar ratio [3]. There are two prerequisites for the formation of such poly(cation-adj-)s with precisely controlled sequences: 1. formation of cationic/aromatic complex by cation– interaction in the precursor solution of polymerization, and 2. the same reactive vinyl head (R1 = R2) of cationic and aromatic monomer pairs.
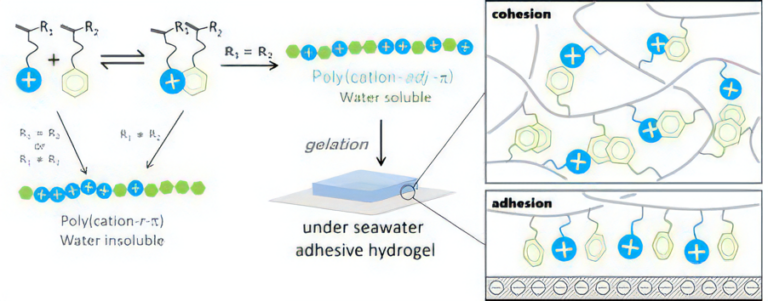
The poly(cation-adj-π) series with adjacently located cationic and aromatic residuals is water-soluble and can form physical hydrogels in seawater.
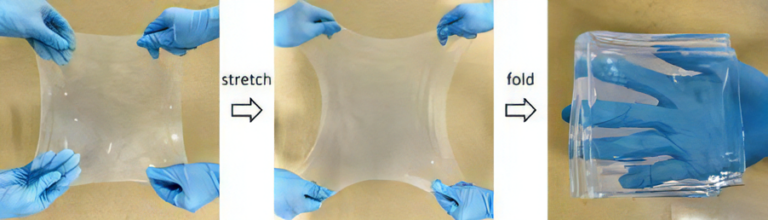
The hydrogels are strong and self-recoverable, exhibiting strong but reversible adhesion to negatively charged surfaces in seawater. The adhesive strength approached approximately 60 kPa with adhesion energy of approximately 30 J/m2. For instance, the poly(2-(acryloyloxy)ethyl trimethyl ammonium chloride-adj-2-phenoxyethyl acrylate) (P(ATAC-adj-PEA)-0.1) gel with a 10-mm diameter and 1.24-mm thickness can rapidly adhere to a 0.49-kg glass block submerged in seawater, and the block can be lifted off of the seawater to air without failure of the adhesion (Video).
Our work provides a route to the synthesis of sequence-controlled polymers by simple free-radical polymerization. The synthesized polymers with adjacent cationic–aromatic sequences not only provide the foundation for the development of adhesives working in saline water such as physiological and marine environments but also give opportunities to study the electrostatic interactions in high ionic-strength conditions. This work may also enable researchers to re-recognize the importance of monomeric sequences toward the properties of materials, which has often been overlooked during material development.
References
- Stuart McLaughlin, Jiyao Wang, Alok Gambhir, D. Murray, PIP2 and Proteins: Interactions, Organization, and Information Flow.Annu. Rev. Biophys. Biomol. Struct. 2002, 31, 151-175.
- J.-F. Lutz, M. Ouchi, D. R. Liu, M. Sawamoto, Sequence-Controlled Polymers.Science 2013, 341, 1238149.
- H. L. Fan, J. H. Wang, Z. Tao, J. C. Huang, P. Rao, T. Kurokawa, J. P. Gong, Adjacent cationic–aromatic sequences yield strong electrostatic adhesion of hydrogels in seawater.Nature Communications 2019, 10, 5127.
Related News
-
博士研究員 Liao HONGGUANGさんと龔先生が発表した Nature 論文が、Nature > News & Views における「Highlights」に選出されました。
- Awards & Publications
-
Mr. Hongguang Liao received Award at Pacifichem 2025.
- Awards & Publications
-
D3 Liao HONGGUANGさんと龔先生が発表したNature論文の内容が様々なメディアで取り上げられました。(9/10追加)
- Media
-
A paper about AI-driven creation of the super-adhesive hydrogels has been published in Nature!
- Awards & Publications
-
Dr. Hailong Fan got Silver Award in The 10th Hokkaido University Cross-Departmental Symposium!
- Awards & Publications
-
[Press Releas] Getting glued in the sea
- Research

