We have developed a hydrogel that does the opposite of what polymer-based materials, like plastic bottles, normally do: this new hydrogel material hardens 1800-fold when heated and softens when cooled [1]! The findings of this work could lead to the fabrication of protective clothing items for traffic and sports-related accidents!
1. Thermal response of general polymers
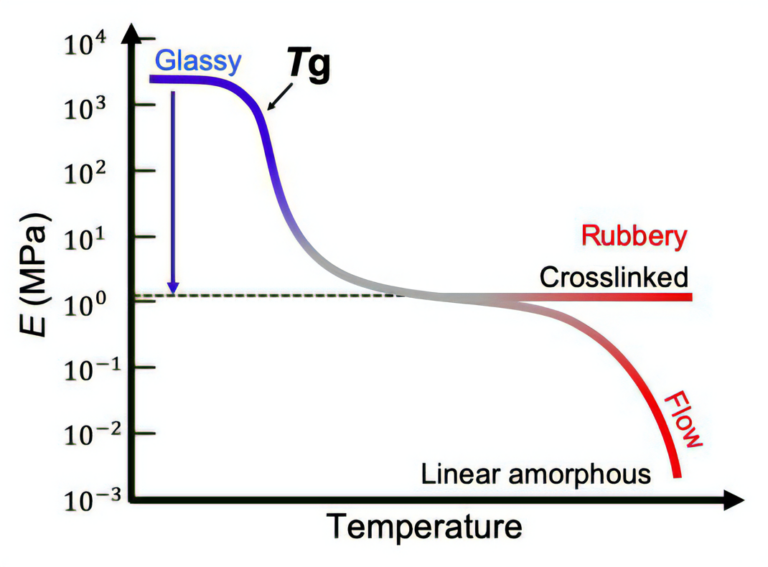
In general, polymeric materials are stiff and soft at low and high temperatures respectively, so that plastic products in daily life, such as plastic bottles and bags, melt and soften when heated. The soft and easily deformable state and the hard and undeformable state are called the “rubbery state” and “glassy state”, respectively, and the transition temperature between these two states is called the “glass transition temperature” (Tg). Figure 1 shows the elastic modulus (hardness) of a polymer over a general temperature range, which varies depending on chemistry [2]. As the temperature is elevated, the modulus drops by approximately three orders of magnitude around the Tg, corresponding to the softening of the plastic. Generally, this three-digit hardness change is almost independent on the chemical species of the polymer. In other words, this is a universal, intrinsic property of polymers. The softening property at high temperatures is very important in the processing of plastic products but has restricted some specific applications of polymers.
2. Strong phase separation inspired by the protein structure of thermophiles
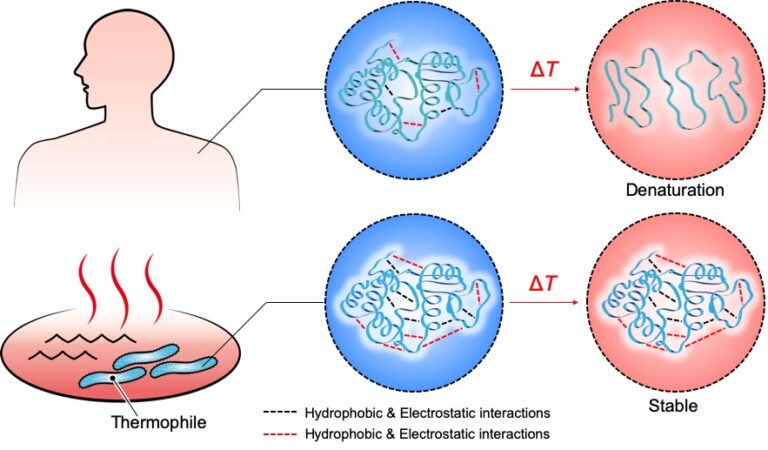
Contrary to the intrinsic property of polymers, we focused on creating hydrogels that exhibit thermal phase separation at high temperatures in order to achieve a rubbery-to-glassy transition with increasing temperature. Phase separation is a phenomenon in which a homogeneous, single-phase substance is divided into two or more phases. In polymer-based hydrogels, the homogenous state, where water and polymer are uniformly distributed, changes to two phases, consisting of polymer dense (dehydrated) and dilute (water-swollen) regions, with increasing temperature [3]. When the gel is phase-separated, the locally formed polymer-rich phase with low water content has more intermolecular interactions, making the gel stiffer. Many polymeric gels exhibit phase separation at high temperatures, but a gel that undergoes phase separation in such a way that vitrification (glass formation) in the polymer-rich phase occurs is a new breakthrough with many potential applications. To achieve strong phase separation, we studied the structure of thermophilic proteins (Fig. 2). Thermophiles are a kind of organism that inhabits hot springs and deep-sea hydrothermal vents, and have adapted to life in high-temperature environments (up to 120°C). Since thermophilic proteins have more ion binding sites and stronger hydrophobic interactions than those of organisms that live in moderate environments like humans, they do not denature at high temperature [4]. In particular, it is known that the strength of ionic bonds is enhanced in hydrophobic environments [5]. Utilizing this principle, we designed a molecule that allows ionic bonds to be incorporated into the polymer dense phase of the phase-separated gel, resulting in glass formation through a process called vitrification.
3. Thermophile-Inspired Hydrogel showing quick thermo-stiffening
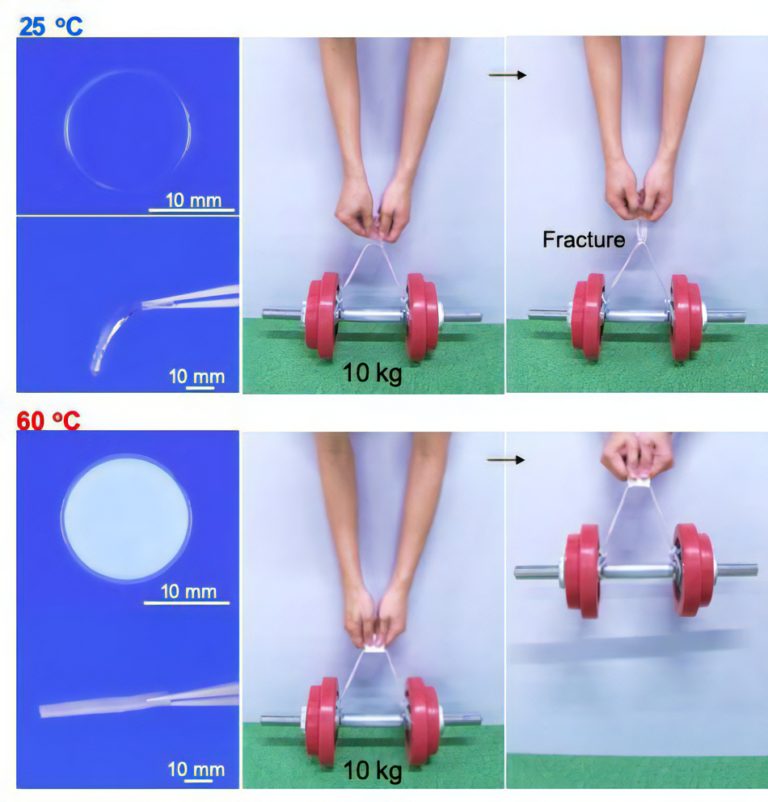
The hydrogel, easily prepared from non-toxic and inexpensive polyacrylic acid and calcium acetate, is transparent and soft in the rubbery state at low temperatures. With increasing temperature, the gel quickly becomes cloudy and changes to an extremely hard, glassy state accompanying phase separation (Figure 3, Movie 1). A folded gel sheet with 1-mm thickness cannot lift up a 10 kg weight at low temperatures, but after phase separation, an 1,800-fold increase in modulus occurs, and the weight can now be supported. This modulus jump is comparable to turning soft jelly into hard plastic. This thermal response is completely reversible, and the hardening temperature can be tuned in the range of 40 to 100 °C, depending on the concentration of polyacrylic acid and calcium acetate.
4. Potential applications as frictional heat responsive protector and thermal absorber
We conducted two practical demonstrations making use of the thermal stiffening behavior of these hydrogels. First, we prepared a thermo-responsive smart protector that is capable of protecting the body and clothes when hardened by frictional heating in traffic and sports incidents. We achieved these properties by fabricating a gel/fabric composite from gel polymerized in the presence of fabric [6]. When the thermo-stiffening gel/glass fiber fabric composite was dragged against the asphalt surface for 5 seconds at a rate of 80 km/h, the surface temperature of the composite immediately rose to 90oC, high enough to cause the hardening transition, and the composite experienced less damage as a result. (Movie 2, Figure 4)
References
- T. Nonoyama, Y. W. Lee, K. Ota, K. Fujioka, W. Hong, J. P. Gong, Instant Thermal Switching from Soft Hydrogel to Rigid Plastics Inspired by Thermophile Proteins.Adv. Mater. 2019 in press.
- M. Rubinstein, R. H. Colby, Polymer Physics.Oxford University Press, Oxford, UK 2003
- K. Mochizuki, D. Ben-Amotz, Hydration-Shell Transformation of Thermosensitive Aqueous Polymers.J. Phys. Chem. Lett. 2017, 8, 1360.
- S. Basak, R. P. Nobrega, D. Tavella, L. M. Deveau, N. Koga, R. Tatsumi-Koga, D. Baker, F. Massi, C. R. Matthews, Networks of electrostatic and hydrophobic interactions modulate the complex folding free energy surface of a designed βα protein.Proc. Natl. Acad. Sci. USA 2019, 116, 6806.
- P. Linse, V. Lobaskin, Electrostatic Attraction and Phase Separation in Solutions of Like-Charged Colloidal Particles.Phys. Rev. Lett. 1999, 83, 4208.
- D. R. King, T. L. Sun, Y. W. Huang, T. Kurokawa, T. Nonoyama, A. J. Crosby, J. P. Gong, Extremely tough composites from fabric reinforced polyampholyte hydrogels.Mater. Horiz. 2015, 2, 584.

