Hydrogels bear some similarities to biological tissues, for example their soft and hydrated form, and hence have been investigated as synthetic equivalents for use in biological systems. Previously they were considered as a class of mechanically weak materials, and thus little attention was paid to them as possible structural materials. For this, and any application as loadbearing materials, hydrogels require a combination of mechanical properties including stiffness, strength, fatigue resistance, damping and self-healing, in addition to a high toughness. Synthesis of new hydrogel materials with these multiple mechanical properties is an important aim in the field. To improve this problem, our laboratory succeeded to present a new class of tough and viscoelastic hydrogels from linear polyampholytes (PA) that can be tuned to change multiple mechanical properties over wide ranges. This hydrogel shows following excellent properties.
Toughness and viscoelasticity
These physical hydrogels are synthesized by random copolymerization of oppositely charged ionic monomers around the charge balance point at high concentration. As one typical example of such a synthesis, we used a pair of ionic monomers, sodium p-styrenesulphonate (NaSS) and 3-(methacryloylamino)propyl-trimethylammonium chloride (MPTC).
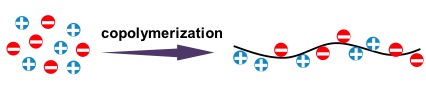
Studying on Double Network (DN) hydrogels concept revealed a new strategy for designing high-strength materials: incorporating, on purpose, a mechanically fragile structure to toughen the material as a whole. As the rupture of the brittle network causes permanent damage, a double-network gel softens and does not recover after experiencing large deformation. To address this problem, the novel tough hydrogels with self-healing features were designed by replacing the covalent bonds with non-covalent bonds to allow the fractured bond to be re-formed. The synthesized linear PA gels can realize this concept. The randomness of charges forms multiple ionic bonds with a wide distribution of strengths, through inter and intrachain complexation. The ionic bonds play two roles for the mechanical properties of the hydrogels: as strong bonds and weak bonds, different from other hydrogels in which they are used as weak bonds. The linear PA hydrogels become tough because they have a different topological structure: the strong bonds form a primary network and the weak bonds form a sacrificial network. The strong bonds serve as permanent crosslinks to maintain the shape of the gel, whereas the weak bonds perform several mechanical functions simultaneously: enhance the fracture resistance by bond rupture and therefore toughen the materials; enhance the shock absorbance by generating high internal friction; enhance the fatigue resistance and self-healing by bond re-formation.
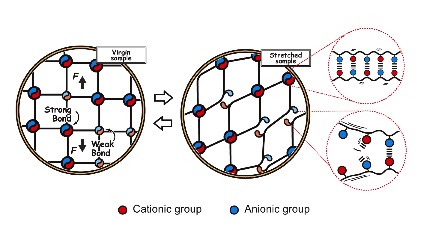
PA gels have a supramolecular structure, and contain 50 ~ 70 wt% of water at an equilibrium state, which is much less than that of conventional hydrogels that usually have a high water content (>80 wt%). They are strongly viscoelastic and have a high toughness (fracture energy of 4,000 Jm-2), 100% self-recovery and high fatigue resistance. Regardless of the modulus change, the PA gels are very strong and tough. They show tensile fracture stress 0.1~ 2 MPa, fracture strain 150 ~ 1,500%, and work of extension at fracture 0.1 ~ 7 MJm-3. The last of these is at least two orders of magnitude larger than that of a conventional hydrogel with the same water content and is comparable to that of a tough double-network hydrogel. The toughness of PA gels is comparable to those of tough double-network hydrogels, soft tissues and filled rubbers.
Self-healing behaviors
As the features of reversible sacrificial ionic bonds, PA gels show self-healing behavior after cutting the samples. As illustrated in following figure if we cut two disc-shaped P(NaSS-co-MPTC) samples dyed red and blue (1) into half-moon pieces (2) and then pressed the cut surfaces of the red and blue pieces together in hot water (50 ), they merged together within several minutes (3).
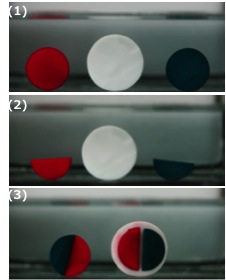
The healing efficiency, defined as the ratio between the work required to break the healing joint and that required to break the virgin sample by tensile deformation, reached ~ 30% after 1 h healing. The healed joint was able to withstand a very large stress. For example, a ribbon of the virgin sample with a 5.2 mm2 cross-section is able to sustain a large load of up to 2 kgf, while the repaired sample can still sustain 0.2 kgf. When the cationic monomer is changed from MPTC to the relatively less hydrophobic methyl chloride quarternized N, N-dimethylamino ethylacrylate (DMAEA-Q), the gel P(NaSS-co-DMAEA-Q) show ~ 99% of self-healing efficiency.
Potential application in hygiene and medical fields
These PA hydrogels have excellent biocompatibility and anti-biofouling properties, as confirmed by the cytotoxicity test and adhesion test using macrophages that are highly adhesive cells responsible for the immune response to implanted materials. As the materials have a wide spectrum of excellent mechanical properties even in physiological solutions, they have high potential as structural biomaterials. Furthermore, the anti-biofouling property suggests potential use in hygiene and medical fields.
References
- Tao Lin Sun, Takayuki Kurokawa, Shinya Kuroda, Abu Bin Ihsan, Taigo Akasaki, Koshiro Sato, Md. Anamul Haque, Tasuku Nakajima, Jian Ping Gong “Physical Hydrogels Composed of Polyampholytes Demonstrate High Toughness and Viscoelasticity” Nature Materials, 12(10), 932-937 (2013).
- Abu Bin Ihsan, Tao Lin Sun, Shinya Kuroda, Md. Anamul Haque, Takayuki Kurokawa, Tasuku Nakajima, Jian Ping Gong “A Phase Diagram of Neutral Polyampholyte – From Solution to Tough Hydrogel” Journal of Materials Chemistiry B, 1(36), 4555-4562(2013).

