
Summary
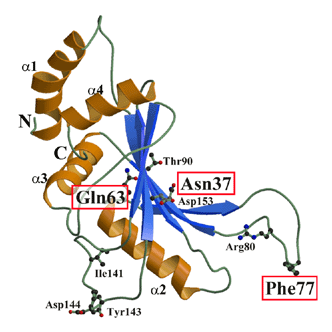
Ribosomal protein L5 is a 5S rRNA binding protein in the large subunit and plays an essential role in the promotion of a particular conformation of 5S rRNA. The crystal structure of the ribosomal protein L5 from Bacillus stearothermophilus has been determined at 1.8 A resolution. The molecule consists of a five-stranded anti-parallel {beta}-sheet and four {alpha}-helices, which fold in a way that is topologically similar to the ribonucleoprotein (RNP) domain. The molecular shape and electrostatic representation suggest that the concave surface and loop regions are involved in 5S rRNA binding. To identify amino-acid residues responsible for 5S rRNA binding, we made use of Ala-scanning mutagenesis of evolutionarily conserved amino acids occurring in the {beta}-strands and loop regions. The mutations of Asn37 at the {beta}1 strand and Gln63 at the loop between helix 2 and {beta}3-strand as well as that of Phe77 at the tip of the loop structure between the {beta}2- and {beta}3-strands caused a significant reduction in 5S rRNA binding. In addition, the mutations of Thr90 on the {beta}3-strand and Ile141 and Asp144 at the loop between {beta}4- and {beta}5-strands moderately reduced the 5S rRNA-binding affinity. Comparison of these results with the more recently analyzed structure of the 50S subunit from Haloarcula marismortui suggests that there are significant differences in the structure at N- and C-terminal regions and probably in the 5S rRNA binding.
References