A Homologue of Eukaryotic Initiation Factor 5A
(eIF-5A)
Eukaryotic initiation factor 5A (eIF-5A) is ubiquitous in eukaryotes and archaebacteria
and is essential for cell proliferation and survival. The crystal structure of the eIF-5A homologue (PhoIF-5A)
from Pyrococcus horikoshii OT3 was determined by the molecular replacement method.
PhoIF-5A is predominantly composed of b-strands
comprising two distinct folding domains, an N-domain and a C-domain,
connected by a short linker peptide (residues 70-71). The N-domain has an SH3-like
barrel, while the C-domain folds in an (oligonucleotide/oligosaccharide binding) OB
fold. Several lines of evidence suggest that eIF-5A functions as a biomodular protein
capable of interacting with protein and nucleic acid. The surface representation of
electrostatic potential shows that PhoIF-5A has a concave surface with positively
charged residues between the N- and C-domains. In addition, a flexible long hairpin
loop, L1 (residues 33-41), with a hypusine modification site is positively charged, protruding
from the N-domain. In contrast, the opposite side of the concave surface at
the C-domain is mostly negatively charged. These findings led to the speculation that
the concave surface and loop L1 at the N-domain may be involved in RNA binding,
while the opposite side of the concave surface in the C-domain may be involved in
protein interaction.
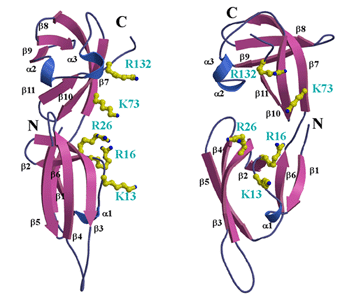
Figure Caption
A cluster of positively charged residues at the putative
RNA-binding region of PhoIF-5A. left: The balls-and-sticks
represent positive charged residues that may contribute to RNA
binding. right: View rotated 90 degree from left one.
References
- Crystal Structure of Hyperthermophilic Archaeal Initiation Factor 5A:
A Homologue of Eukaryotic Initiation Factor 5A (eIF-5A)
Min Yao, Akiko Ohsawa, Shingo Kikukawa, Isao Tanaka and Makoto Kimura
J. Biochem.(ToKyo), 133, 75-81 (2003)
|
