
ABSTRACT
ThiI catalyzes the thio-introduction reaction to tRNA, and a truncated tRNA consisting of 39 nucleotides, TPHE39A, is the minimal RNA substrate for modification by ThiI from Escherichia coli. To examine the molecular basis of the tRNA recognition by ThiI, we have solved the crystal structure of TPHE39A, which showed that base pairs in the T-stem were almost completely disrupted, although those in the acceptor-stem were preserved. Gel shift assays and isothermal titration calorimetry experiments showed that ThiI can efficiently bind with not only tRNAPhe but also TPHE39A. Binding assays using truncated ThiI, i.e., N- and C-terminal domains of ThiI, showed that the N-domain can bind with both tRNAPhe and TPHE39A, whereas the C-domain cannot. These results indicated that the N-domain of ThiI recognizes the acceptor-stem region. Thermodynamic analysis indicated that the C-domain also affects RNA binding by its enthalpically favorable, but entropically unfavorable, contribution. In addition, circular dichroism spectra showed that the C-domain induced a conformation change in tRNAPhe. Based on these results, a possible RNA binding mechanism of ThiI in which the N-terminal domain recognizes the acceptor-stem region and the C-terminal region causes a conformational change of RNA is proposed.
FIGURE CAPTION
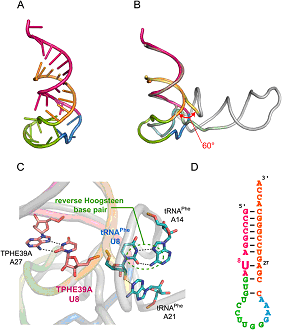
Crystal structure of TPHE39A.
(A) Overall structure of TPHE39A. The acceptorstem regions are colored orange (G1-A9) and pink (C24-C38).
Nucleotides corresponding to the T-stem and the T-loop in tRNAPhe are shown in green (G10-G19) and blue (G20-A23), respectively.
(B) Superposition of TPHE39A and tRNAPhe. Atoms of G1-A9 are used for superposition.
Nucleotides corresponding to the acceptor-stem (G1-A9), acceptor-stem (U66-A76),
T-stem, T-loop, and others are colored pastel yellow, pastel pink, pastel green, pastel blue, and gray, respectively.
(C) Close-up view around U8. U8 and its base-pairing nucleotides in TPHE39A and tRNAPhe are shown as pink and blue sticks, respectively.
Hydrogen bonds are also shown as dotted lines. Colors of other regions correspond to those in B.
(D) Secondary structure of TPHE39A according to the crystal structure.
REFERENCES
Deduced RNA binding mechanism of ThiI based on structural and binding analyses of a minimal RNA ligand.
Yoshikazu Tanaka, Shiori Yamagata, Yu Kitago, Yoko Yamada, Sarin Chimnaronk, Min Yao, and Isao Tanaka.
RNA 15, 1498-1506 (2009)