Monomeric Isocitrate Dehydrogenase from Nitrogen-Fixing Bacterium Azotobacter vinelandii

Summary
NADP+-dependent isocitrate dehydrogenase is a member of the b-decarboxylating dehydrogenase family, and catalyzes the oxidative decarboxylation reaction from 2R,3S-isocitrate to yield 2-oxoglutarate and CO2 in the Krebs cycle. Although most prokaryotic NADP+-dependent IDHs are dimeric enzymes composed of two identical subunits with a molecular weight of 40-50 kDa, the monomeric IDH with a molecular weight of 80-100 kDa has been found in a few species of bacteria. We successfully determined the 1.95 A crystal structure of the monomeric IDH in complex with isocitrate and Mn2+ from the nitrogen-fixing bacterium Azotobacter vinelandii by the multiwavelength anomalous diffraction (MAD) method. The MAD phase calculation was performed with the anomalous signal of two Mn atoms bound to two independent IDH molecules in an asymmetric unit. The final refined model of the monomeric IDH revealed that it consists of two distinct domains, and its folding topology is related to the homodimeric form of the dimeric IDH. The structure of the large domain repeats a motif observed in the dimeric IDH, and the motifs are related by a pseudo 2-fold axis. This pseudo symmetry coincides with a crystallographic 2-fold axis that relates identical subunits of the E. coli dimeric IDH. Such a fusional structure by a domain duplication enables a single polypeptide chain to form a structure at the catalytic site that is homologous to the dimeric IDH, the catalytic site of which is located at the interface of two identical subunits and is formed with residues from both subunits.Figure Caption
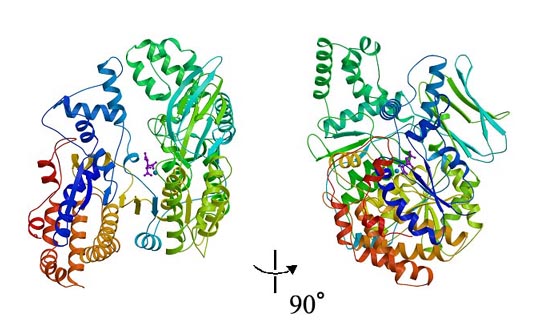
Two perpendicular views ribbon diagram of the monomeric IDH complexed with isocitrate and Mn2+. The model is colored according to sequence by a rainbow color ramp from blue at the N-terminus to red at the C-terminus. The isocitrate molecule is represented as a ball-and-stick model colored in magenta and Mn2+ is represented as a ball model colored in blue
References
| To our projects page |