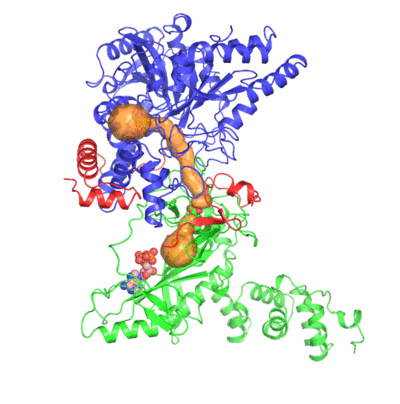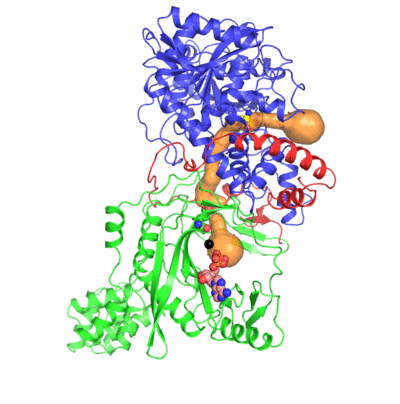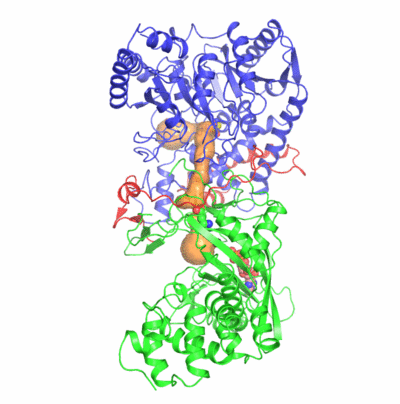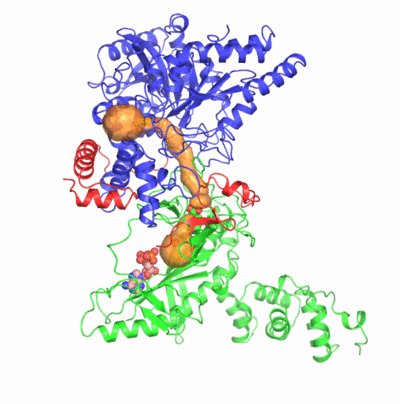
The formation of Gln-tRNAGln differs among the three domains of life.
Most bacteria employ an indirect pathway to produce Gln-tRNAGln by
a heterotrimeric amidotransferase, GatCAB, in a mis-acylated Glu-tRNAGln-dependent
manner. Hampered by the absence of an atomic model, very little is known about
the molecular mechanism responsible for the sophisticated coupling reactions of GatCAB.
Here, we report the three first crystal structures of intact GatCAB complex from
Staphylococcus aureus, in the apo form, the glutamine- and the ATP analog-bound states.
Two identified active sites are markedly distant but connected by an idiosyncratic
ammonia channel 30 angstrom in length. Furthermore, we also clarified identity elements
essential for discrimination of tRNAGln, and propose a complete model for the
overall concerted reactions to generate Gln-tRNAGln by GatCAB.