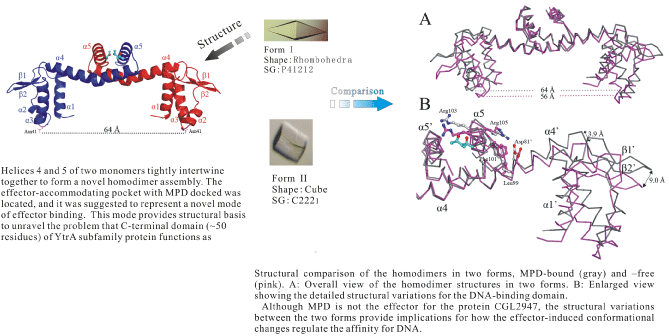

Among the transcription factors, the helix-turn-helix (HTH) GntR family comprised of FadR, HutC, MocR, YtrA, AraR, and PlmA subfamilies regulates the most varied biological processes. Generally, proteins belonging to this family contain an N-terminal DNA-binding domain and a C-terminal effectorbinding/oligomerization domain. The members of the YtrA subfamily are much shorter than other members of this family, with chain lengths of 120?130 residues with about 50 residues located in the C-terminal domain. Because of this length, the mode of dimerization and the ability to bind effectors by the C-terminal domain are puzzling. Here, we first report the structure of the transcription factor CGL2947 from Corynebacterium glutamicum, which belongs to the YtrA family. The monomer is composed of a DNA-binding domain containing a winged HTH motif in the N terminus and two helices (a4 and a5) with a fishhook-shaped arrangement in the C terminus. Helices a4 and a5 of two monomers intertwine together to form a novel homodimer assembly. The effector-accommodating pocket with 2-methyl-2,4-pentanediol (MPD) docked was located, and it was suggested to represent a novel mode of effector binding. The structures in two crystal forms (MPD-free and -bound in the proposed effectorbinding pocket) were solved. The structural variations have implications regarding how the effectorinduced conformational change modulates DNA affinity for YtrA family members.
Reference