
Summary
A pyridoxal 5’-phosphate (PLP)-dependent enzyme 1-aminocyclopropane-1-carboxylate deaminase (ACCD) catalyzes a reaction that involves a ring opening of cyclopropanoid amino acid ACC, yielding α-ketobutyrate and ammonia. Unlike other PLP-dependent enzymes, this enzyme has no α-hydrogen atom in the substrate. Thus, a unique mechanism for the bond cleavage is expected. The crystal structure of ACCD from Hansenula saturnushas been determined at 2.0Å resolution by the multiple wavelength anomalous diffraction (MAD) method using mercury atoms as anomalous scatterers. The model was built on the electron density map, which was obtained by the density averaging of multiple crystal forms. The final model was refined to an R-factor of 22.5% and an Rfree-factor of 26.8%. The ACCD folds into two domains, each of which has an open twisted α/β structure similar to the β-subunit of tryptophan synthase. However, in ACCD, unlike in other members of the β family of PLP-dependent enzymes, PLP is buried deep in the molecule. The structure provides the first view of the catalytic center of the cyclopropane-ring opening.
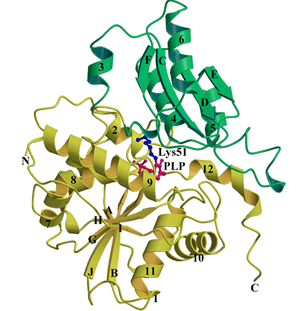
Figure Caption
Ribbon diagram of the yACCD monomer consisting of two domains. Green represents the small domain (residues 58-169) and yellow represents the PLP-binding domain (residues 1 to 57 and 170 to 341). Both domains fold as an open twisted α/β structure consisting of a central β-sheet and surrounding helices. The PLP molecule (red) and Lys51 (blue) are shown as balls-and-sticks.
References
| J. Biol. Chem. 275, 34557-34565 (2000) |
|
| To our projects page |